ECLIPSE PV (NCT05481151): a Phase 3b, randomized, open-label, parallel-group, multicenter study to evaluate the efficacy and safety of two dosing regimens of ropeginterferon alfa-2b-njft (an accelerated titrated dosing versus the current recommended dosing) for adult patients with polycythemia vera (PV).1,2
Ropeginterferon alfa-2b-njft is currently approved by the FDA for the treatment of PV.1
PharmaEssentia is the sponsor of the ECLIPSE PV study.1
Now recruiting: Phase 3b1,2
What is the ECLIPSE PV study?1
PharmaEssentia is conducting a Phase 3b clinical research study in adult patients with PV. The purpose of the ECLIPSE PV study is to evaluate the efficacy and safety of two dosing regimens of ropeginterferon alfa-2b-njft in PV.
Study design (N≈100)
- Adult patients with a confirmed diagnosis of PV according to the 2008 or 2016 WHO criteria
- Does not include patients who stopped prior IFN alfa therapy due to low efficacy or poor tolerability
- See additional eligibility criteria
4 weeks
Screening period
6 months
6 months
Core treatment period
Extension period
Ropeginterferonalfa-2b-njft
Arm 1: 100 mcg SC (Week 0) and titrated up by 50 mcg Q2W*† Arm 2: 250 mcg SC (Week 0), followed by 350 mcg (Week 2), and 500 mcg* (Week 4)
4 weeks
Follow-up period
*
The maximum recommended single dose is 500 mcg SC Q2W. †Patients receiving HU upon enrollment will have a starting dose of 50 mcg at Day 0.
Primary endpoint
- CHR at Week 24 (HCT <45% and phlebotomy free in the prior 12 weeks, PLT count ≤400 x 109/L, and WBC count <10 x 109/L)
Key secondary endpoints
- CHR at Weeks 8, 12, 16, 20, 24, 36, and 48
- Durable response (CHR at Weeks 36 and 48)
- Time to first CHR
- Time to maintenance dose
- Change in HCT, PLT, and WBC counts from baseline
- Time to freedom from phlebotomy for the previous 12 weeks
- Duration of peripheral blood cell count (HCT, PLT, and WBC) response
- Improvement in symptoms using the MPN-SAF TSS
- Proportion of subjects without thrombotic or hemorrhagic events at Weeks 8, 12, 24, 36, and 48
- Tolerability of ropeginterferon alfa-2b-njft
Exploratory endpoint
- Bone marrow histological remission
What is polycythemia vera (PV)?
PV is a rare, chronic myeloproliferative neoplasm characterized by erythrocytosis, leukocytosis, and thrombocytosis.3 In over 95% of cases, PV is driven by a mutation in the JAK2 gene.4 This mutation leads to the over proliferation of hematopoietic stem and progenitor cells in the bone marrow, resulting in thickening of the blood due to an excessive number of cells and a persistent threat of hemorrhage, thrombosis, cardiovascular events, and other debilitating symptoms.5-7 Current therapies used to treat PV may include HU, IFN alfa, and ruxolitinib (an FDA-approved therapy for those who do not respond to or cannot tolerate HU).3
What is ropeginterferon alfa-2b-njft?
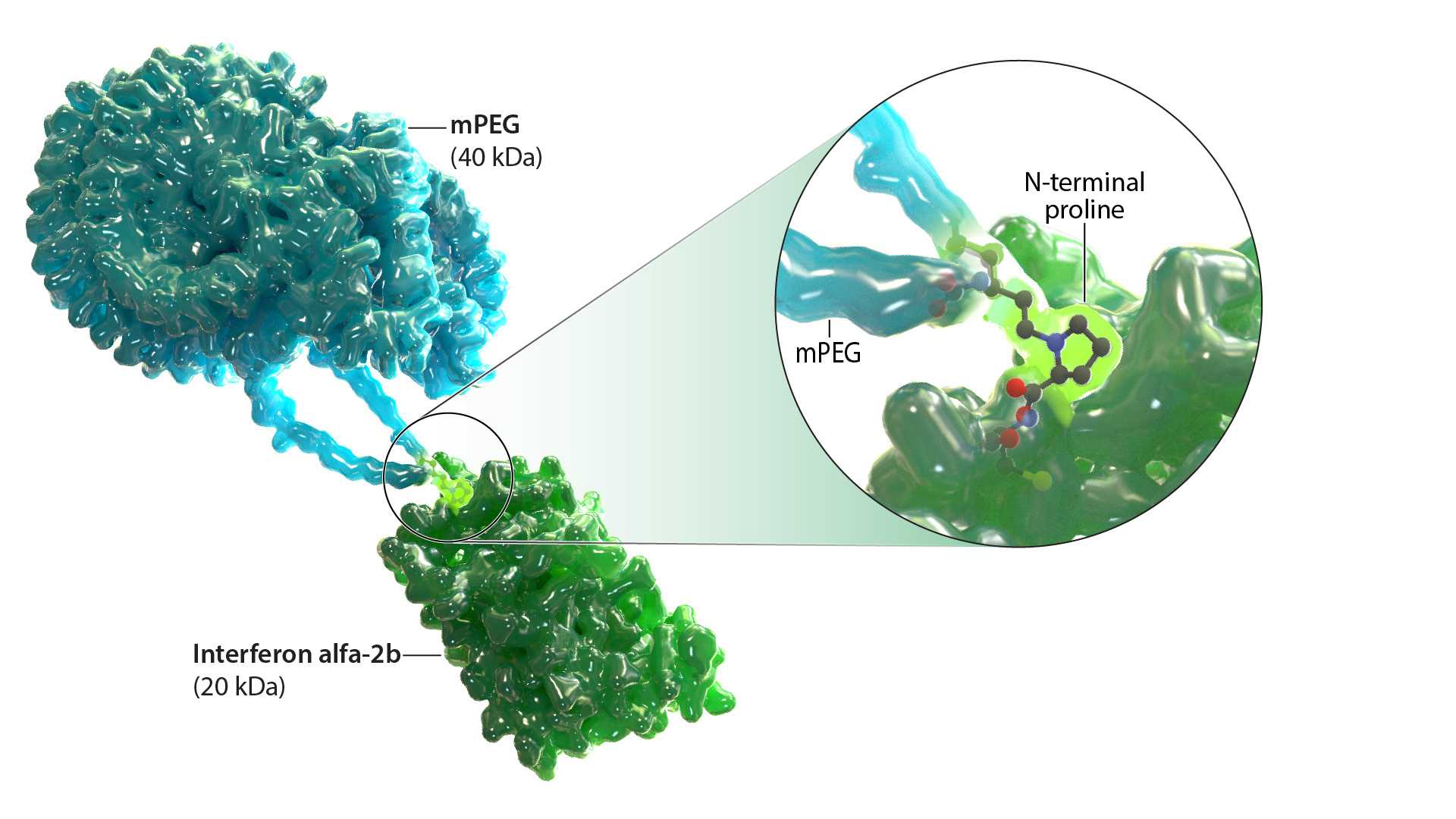
Ropeginterferon alfa-2b-njft is a monopegylated IFN alfa-2b that selectively targets JAK2 mutant hematopoietic stem and progenitor cells.8,9 It is currently approved by the FDA for the treatment of PV.1
Who is eligible for ECLIPSE PV?1
≥18 years of age
Confirmed diagnosis of PV according to the 2008 or 2016 WHO criteria
Not refractory or intolerant to IFN alfa
Neutrophil count ≥1.5 x 109/L
CrCl ≥40 mL/min
Adequate hepatic function (total bilirubin ≤1.5 x ULN, INR ≤1.5 x ULN, albumin >3.5 g/dL, ALT ≤2.0 x ULN, and AST ≤2.0 x ULN)
Hemoglobin ≥10 g/dL for females and ≥11 g/dL for males
For additional information on eligibility criteria, visit ClinicalTrials.gov (trial identifier: NCT05481151).2
Where is ECLIPSE PV being conducted?
Find out if there’s a study site in your area using the table below. For additional information, please contact us by emailing clinicaltrials@pharmaessentia-us.com or call 1-800-999-2449.
- St. Paul's Hospital
- Vancouver, BC
- Juravinski Cancer Centre
- Hamilton, ON
- Princess Margaret Hospital
- Toronto, ON
- John Theurer Cancer Center at Hackensack UMC
- Hackensack, NJ
- Mount Sinai Medical Center
- New York City, NY
- Fox Chase Cancer Center
- Philadelphia, PA
- American Oncology Partners of Maryland PA (Center for Cancer & Blood Disorders)
- Bethesda, MD
- MD Anderson Cancer Center
- Houston, TX
- University of Virginia - Emily Couric Cancer Center
- Charlottesville, VA
- University of Utah
- Salt Lake City, UT
- University of Michigan
- Ann Arbor, MI
- Washington University School of Medicine - Division of Oncology
- St. Louis, MO
How do I refer patients to ECLIPSE PV?
To refer a patient, please contact our study information center at 1-800-999-2449.
To become an investigator or conduct the ECLIPSE PV study at your institution, please contact our Medical Information team at medinfo@pharmaessentia-us.com.